Cycloalkanes which are also known as cycloparaffins are saturated hydrocarbons. Cyclopropane, Cyclobutane, Cyclopentane, Cyclohexane etc. are the examples of cycloalkanes.
Cyclopropane and Cyclobutane are present as in gaseous state at room temperature and other remaining cycloalkanes are exist in liquid state. Cycloalkane resemble alkanes in their chemical behaviour while cyclopropane and cyclobutane are exceptions.
Ring-opening reactions-
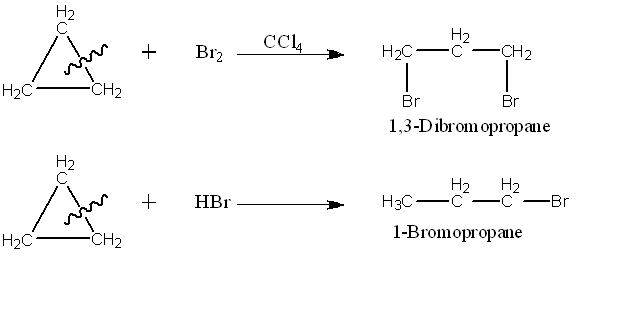
- Cyclopropane reacts with chlorine and bromine in the dark to form addition products. Cyclobutane and higher cycloalkanes do not give this ring opening reaction.
- Cyclopropane also react with HBr to form addition product. Cyclobutane and higher cycloalkanes do not give this ring opening reaction.
So, why cyclobutane and higher cycloalkanes do not give ring opening reactions. This can be explained by the basis of angle strain theory. Any deviation of bond angles from the normal tetrahedral value would impose a condition of internal strain on the ring.

In cyclopropane, C-C-C bond angle is 60°. This revealed that normal tetrahedral angle of 109°28′ between any two bonds is compressed to 60°. Angle strain or deviation is calculated as 24°44′. Same as angle strain for other cycloalkanes can be calculated. The angle strain is maximum in the case of cyclopropane. According to “Baeyer strain theory”, cyclopropane is highly strained molecule and found most unstable. Thus, cyclopropane ring should be expected to open up on slightest provocation and thus releasing the strain within it.