In nature, there are two category of chemical compounds mainly, Inorganic and Organic. Inorganic compounds are derived from mineral origin. Long time ago, it was believed that organic compounds are derived only animal or vegetable sources. The word “organic” closely related to “organism”. According to Berzelius, Vital Force Theory, “the living matter could be only source of organic compounds due to having vital force.” As long as this theory prevailed no effort was made to produce organic compounds in the laboratory, and the Vital Force Theory long went unchallenged.
After a long time, the German scientist Friedrich Wohler synthesized the Urea from inorganic compound Ammonium Cyanate in the laboratory. The Urea found in this way proved to be identical in all perspective with urea isolated from urine.
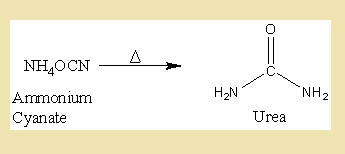
After this there were many organic compounds synthesized in the laboratory and thus the vital force was finally and decisively disproved.
Organic Chemistry in Modern Perspectives
Organic chemistry is the study of carbon compounds. The number of compounds that contain carbon is many times greater than the number of compounds that do not contain carbon. Organic molecules containing thousands of atoms are known, and the arrangement of atoms in even relatively small molecules can be very complicated.
Organic chemistry is very fundamental to biology and medicine because living organisms are made up of chiefly organic molecules such as carbohydrates, proteins, lipids that are also known as Biomolecules.
Why Carbon has larger number of compounds?
Carbon can attach themselves to one another atom to an extent not possible for atoms of any other element. Carbon can form chains thousands of atoms long, or rings of all sizes; the chains and rings can have branches and cross-links. This ability of carbon to bond successively to other atoms to form chains or rings of varying lengths and shapes is defined as Catenation. Unique chemical and physical characteristics and Catenation are responsible for the large number of organic compounds.
Why CO2, CO, CS2, KCN, NaSCN are not considered as organic compound?
| Bond | Bond energy (kcal/mole) |
| C-H | 99 |
| C=O | 179 |
| C-O | 86 |
| C-C | 83 |
| C=C | 146 |
| N-H | 93 |
Organic compounds are not only carbon containing compounds. Organic compounds are the hydrocarbons or derivatives of hydrocarbons. So, when we determine whether a carbon containing molecule is organic or not, must see whether carbon forms bond with hydrogen or whether molecule contains hydrogen in addition to carbon. We also look to see what type of bond is formed and bond energy, bond length considered. In CO2 molecule, C-O bond energy is higher as compared to C-H bond. This higher bond energy stabilizes the molecule and less reactive.


Nice information..
Pls also read:
“Exploring Everyday Chemistry” 4 week informative free certificate™ course from University of York from futurelearn
LikeLike